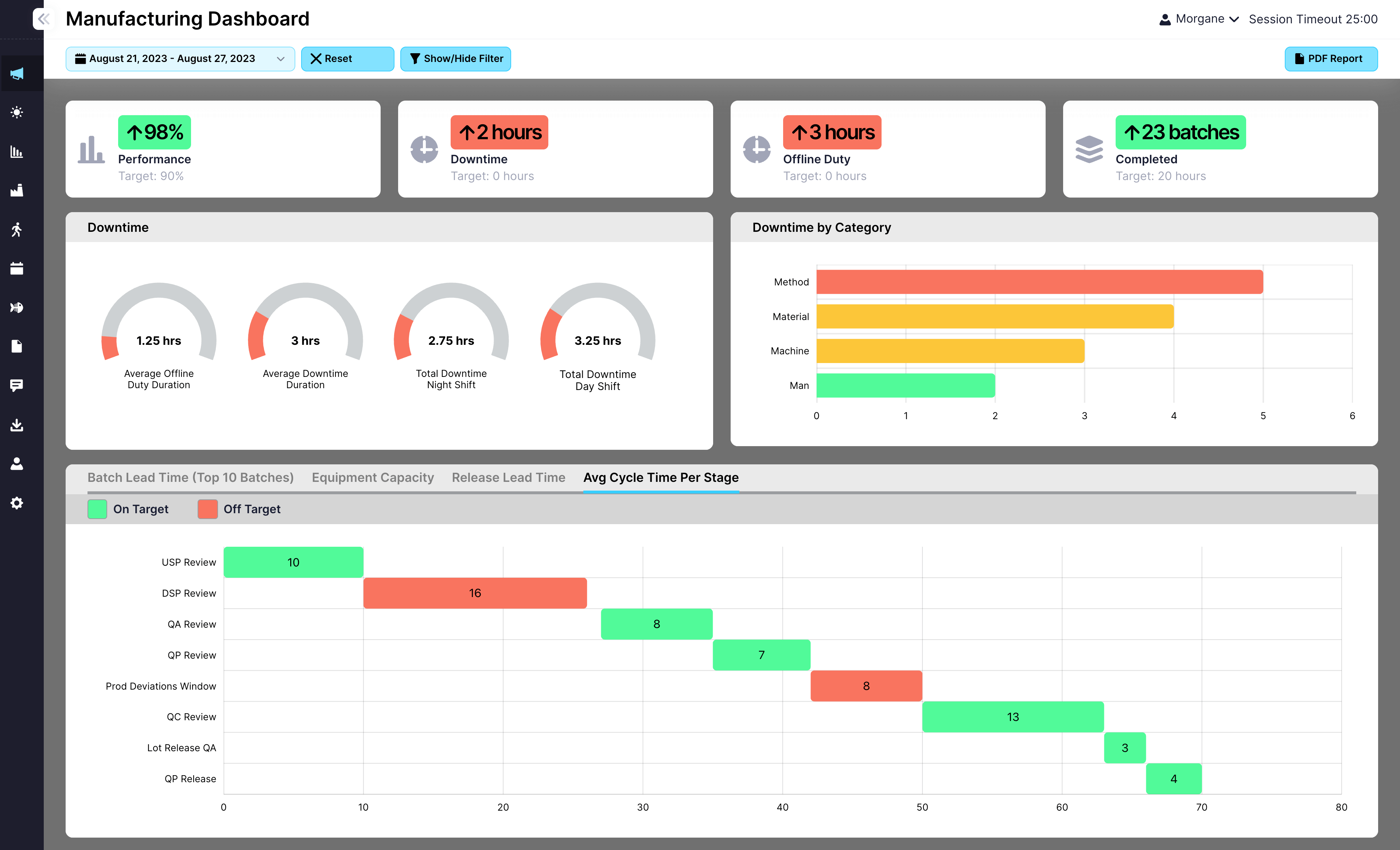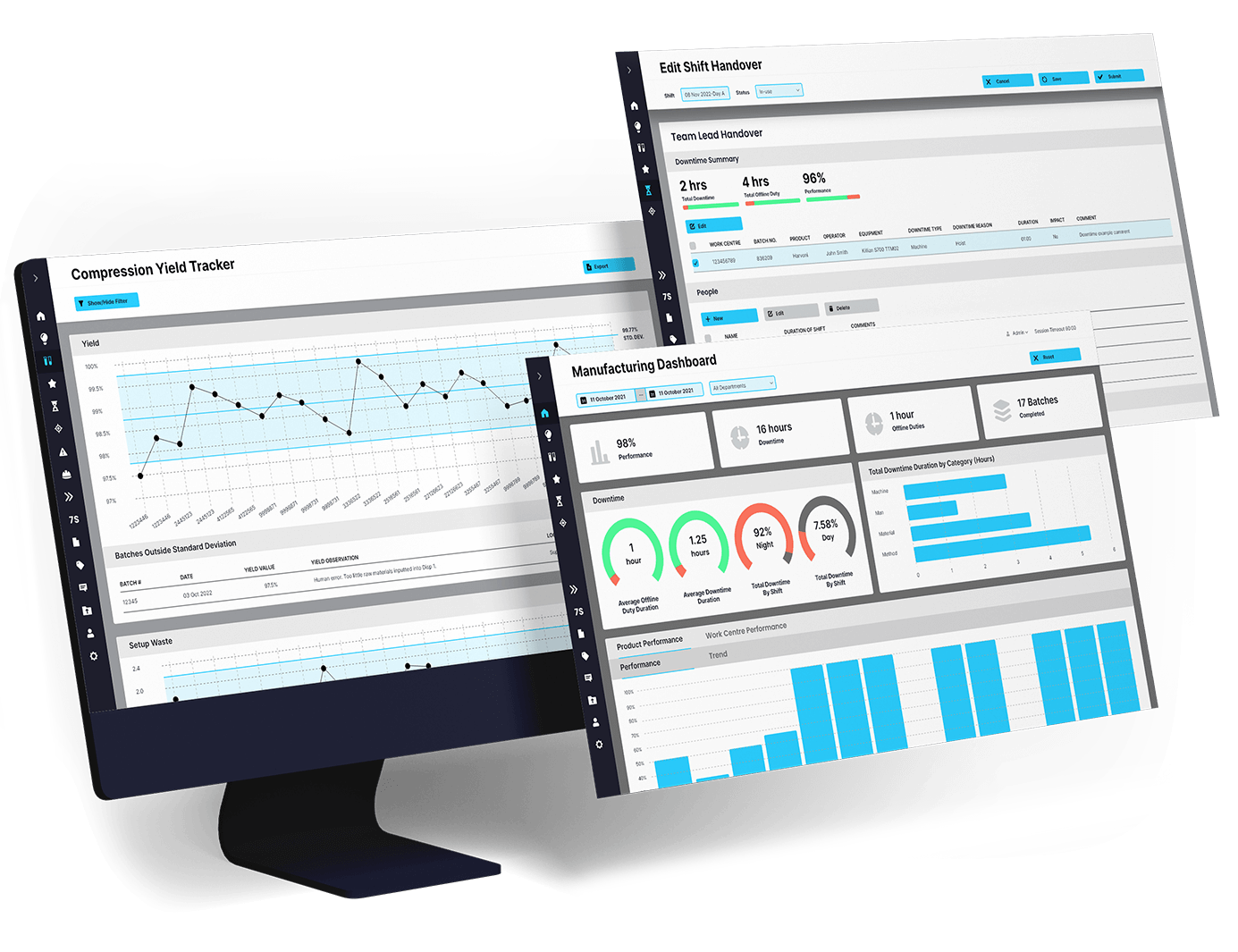
EviView’s EHS reports give safety teams a real time view of incidents, inspections, audits, and follow-up tasks. No more chasing down spreadsheets or waiting on monthly summaries. With all your safety data in one place, you can act fast, see what is improving, and stay audit ready at all times.
Request a DemoEviView brings together all safety data in one connected system so teams can see what has happened, what is in progress, and what needs attention next.
View reports for incidents, inspections, actions, and risk assessments
Filter by location, team, timeframe, or event type
Export data easily for audits, meetings, or compliance reviews
Connect reports to dashboards, KPIs, and team-level visibility

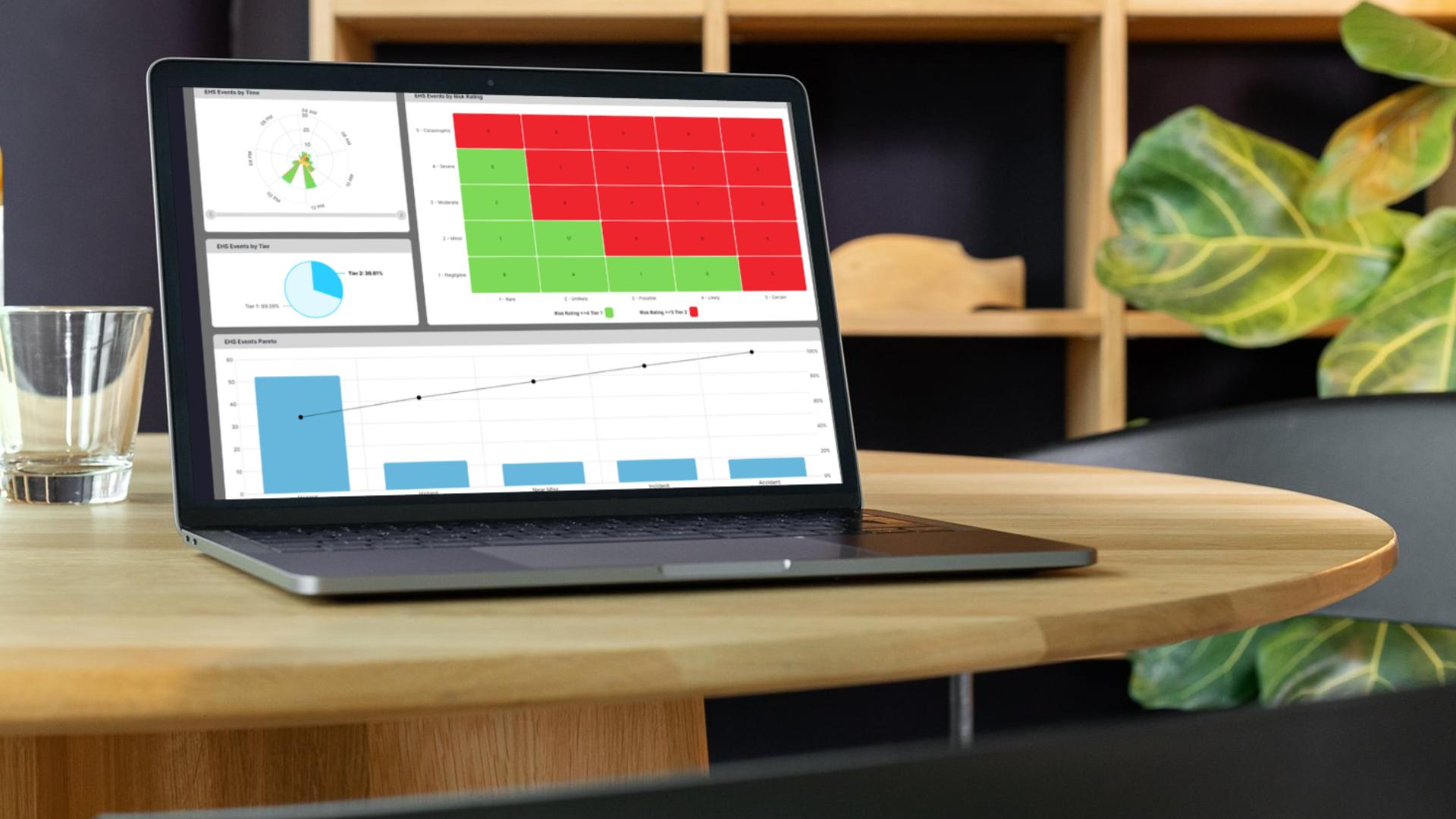
See incidents, inspections, and follow-ups together in one reliable system.
Use live updates to fix problems and reduce delays in safety action.
Export reports and show progress clearly to auditors, customers, and leadership.
See which areas are improving and where more attention is needed across teams.
Without clear EHS reporting, teams work in the dark. EviView gives you accurate, real time updates from the floor. Whether reviewing trends or preparing for audits, safety leaders get the data they need to respond quickly, prove progress, and close the loop on every safety action.
EviView’s EHS reports work with the systems you already use, like QMS, ERP, and production tools. Report data flows into dashboards, audits, and team reviews without double entry or extra admin. That means better visibility, smoother compliance, and faster decisions across every level of the operation.
Request a Demo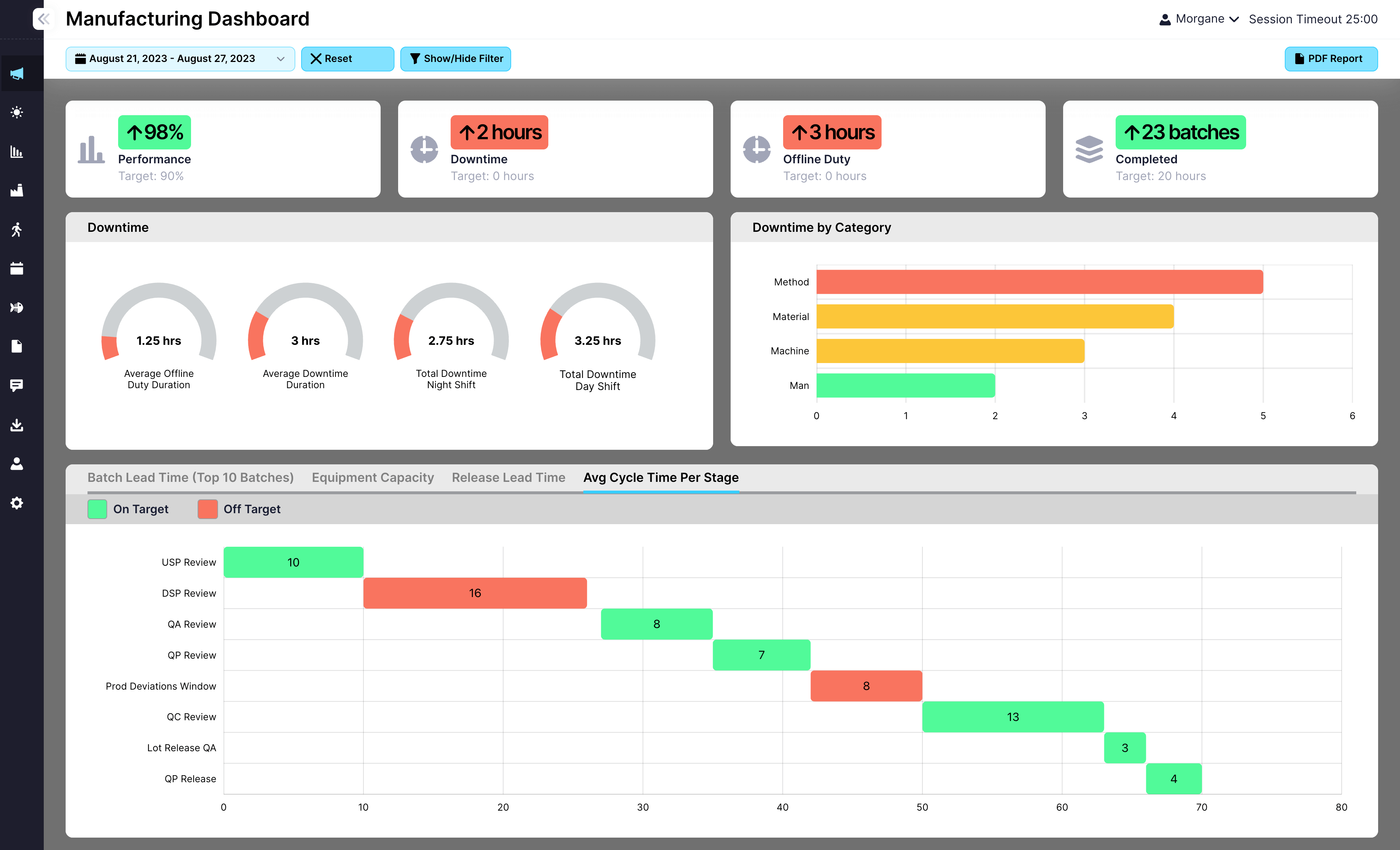
Here is how real safety teams use EviView’s EHS reports to stay organized, reduce response time, and lead improvements across shifts, departments, and sites.

EviView’s Digital Daily Management System has been transformative for Merck. By streamlining shift handovers, centralizing data, and integrating with SAP and Palantir Data Lake, we’ve reduced downtime by 30% and saved €66,000 annually. The system has boosted visibility, improved communication, and increased safety incident reporting by 300%, fostering a culture of continuous improvement and positioning us for long-term success.

Pharmaceutical Company, US
Head of Digitalisation and Strategy

I worked with EviView on an electronic handover project. Their team was very knowledgeable, took time to ensure all stakeholders were kept up to date with the progress of the project, and was always available to give advice on system usage during implementation.

Bio-Pharma Company, US
Manufacturing Team Lead

Karol's team delivered a highly successful change management and digital transformation project. His structured approach, detailed analysis, and process-driven methodology have been invaluable on every project. EviView's understanding of business, IT, operations, and budgetary needs is unmatched.

Top 5 Pharma Manufacturer, EU
Senior Manager of Engineering & Maintenance
EviView turns safety reporting into a powerful tool for progress. Instead of disconnected forms or static PDFs, teams get real time visibility and proof that actions are making a difference. Every report builds confidence, every trend guides smarter planning, and every action taken moves safety forward.