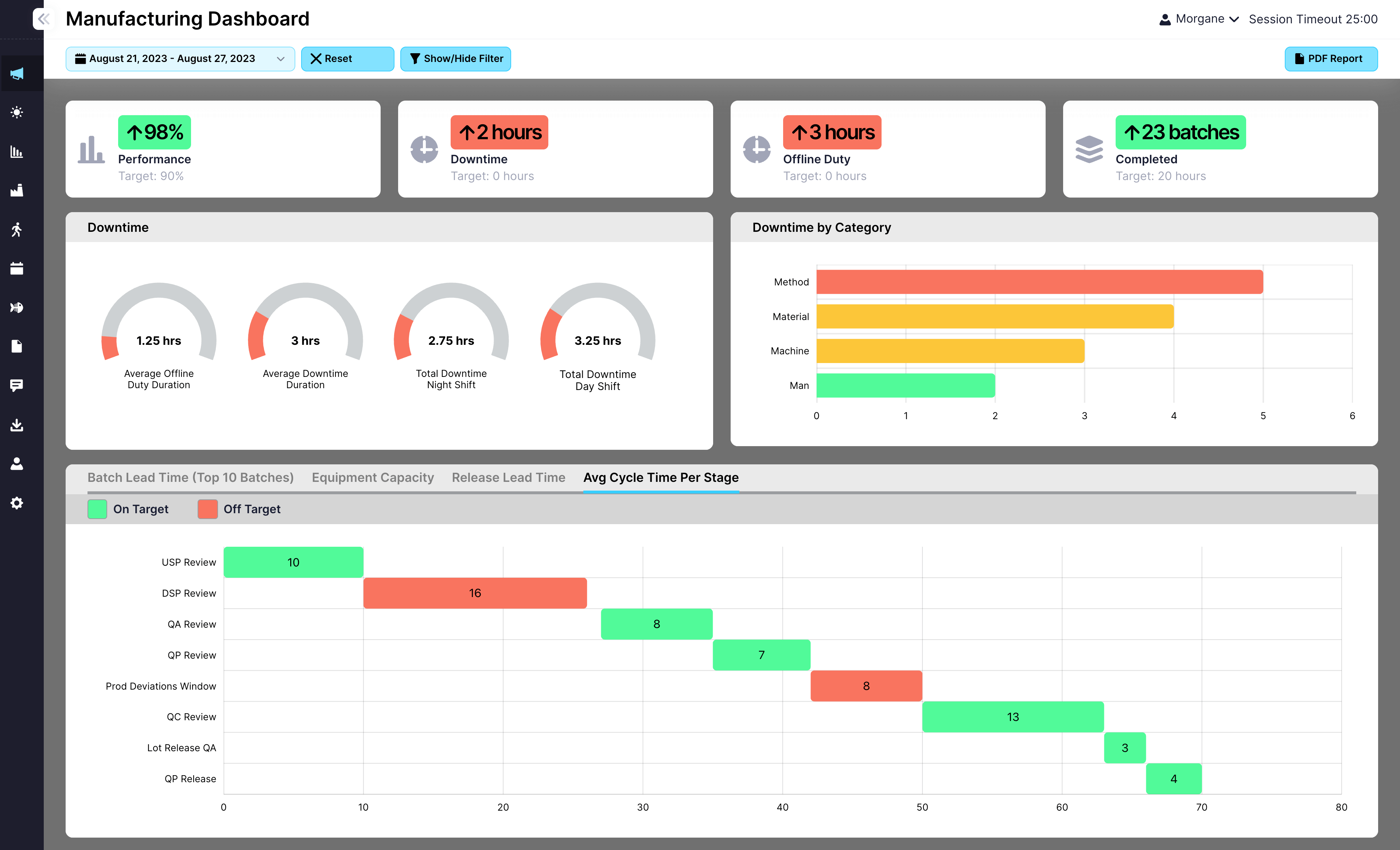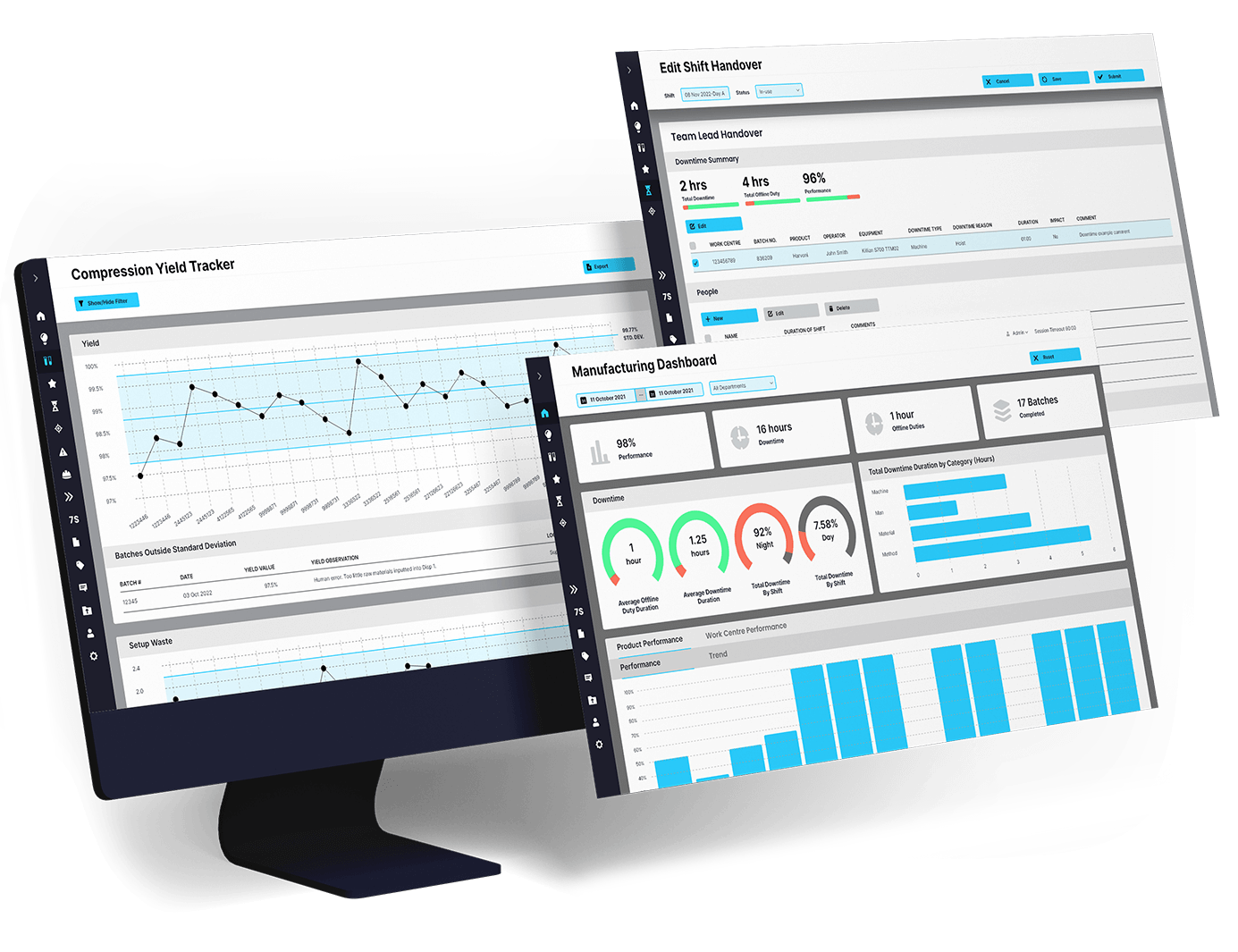
EviView’s risk assessments help teams identify, evaluate, and manage safety risks directly from the floor. From routine checks to high-risk tasks, you can document hazards, assign controls, and track follow-ups in one place. No spreadsheets. No delays. Just clear actions and real accountability at every stage.
Request a DemoEviView makes risk assessments part of daily safety routines. Teams complete assessments on-site, capture evidence, and assign controls with full visibility for EHS leads.
Document hazards by task, location, or equipment
Assign controls and follow-up actions immediately
Link assessments to incidents, audits, or permits
Track status and ownership across departments and shifts

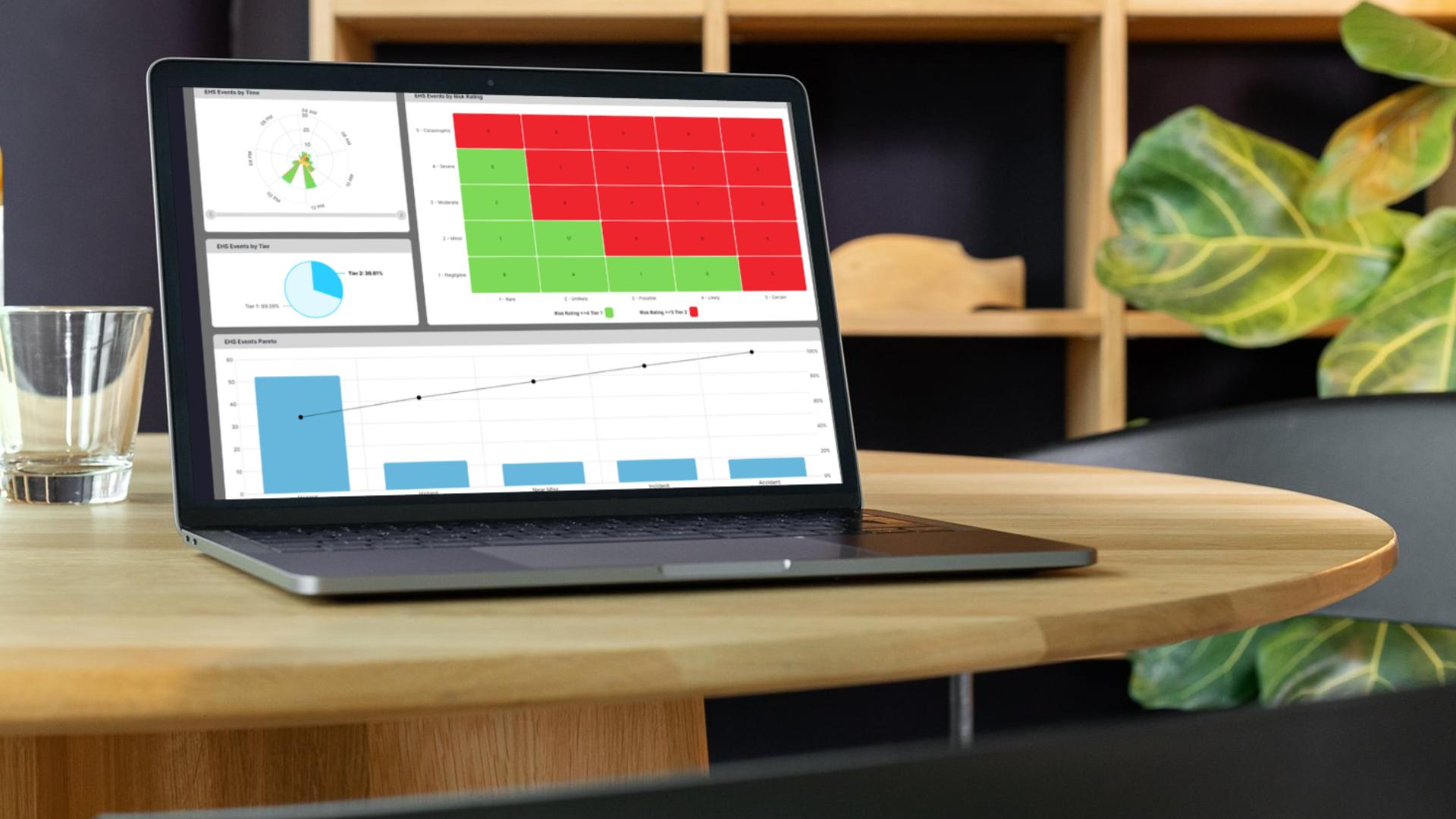
Spot potential hazards early and assign controls directly from the floor.
Assign ownership and due dates so tasks are never missed or forgotten.
Keep documentation complete and accessible with real-time reporting and history.
Automate tracking and reduce admin time while improving accountability.
When risk assessments are scattered or outdated, hazards go unmanaged and teams lose trust in the process. EviView brings structure, clarity, and speed. From identifying risks to verifying control actions, everything is visible. Safety teams can follow through, escalate fast, and show that risk is being managed effectively every day.
EviView connects with your existing EHS, QMS, and production tools to keep safety work flowing. Risk assessments and follow-ups feed into your existing reports and systems. No manual tracking. No duplication. Just one connected workflow that gives teams confidence and gives leaders a real view of risk management progress.
Request a Demo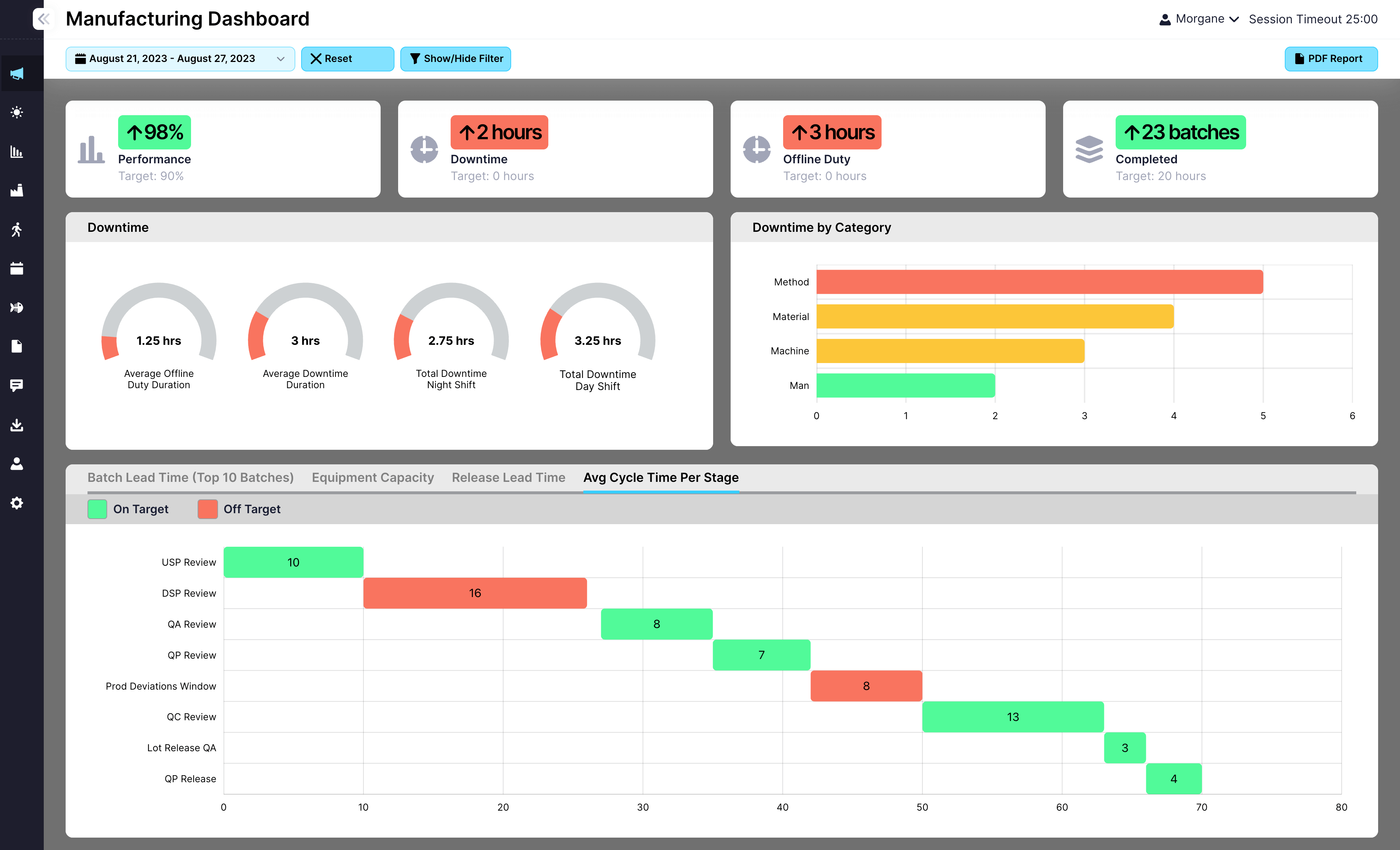
Here is how real teams use EviView’s risk assessments to manage hazards clearly, assign responsibility, and keep safety work moving.

EviView’s Digital Daily Management System has been transformative for Merck. By streamlining shift handovers, centralizing data, and integrating with SAP and Palantir Data Lake, we’ve reduced downtime by 30% and saved €66,000 annually. The system has boosted visibility, improved communication, and increased safety incident reporting by 300%, fostering a culture of continuous improvement and positioning us for long-term success.

Pharmaceutical Company, US
Head of Digitalisation and Strategy

I worked with EviView on an electronic handover project. Their team was very knowledgeable, took time to ensure all stakeholders were kept up to date with the progress of the project, and was always available to give advice on system usage during implementation.

Bio-Pharma Company, US
Manufacturing Team Lead

Karol's team delivered a highly successful change management and digital transformation project. His structured approach, detailed analysis, and process-driven methodology have been invaluable on every project. EviView's understanding of business, IT, operations, and budgetary needs is unmatched.

Top 5 Pharma Manufacturer, EU
Senior Manager of Engineering & Maintenance
EviView turns every assessment into a trackable process. Hazards are documented, controls assigned, and actions followed through. If something gets missed, the system flags it. If issues repeat, the data is there. This is risk management built for teams that need to act fast and prove it later.