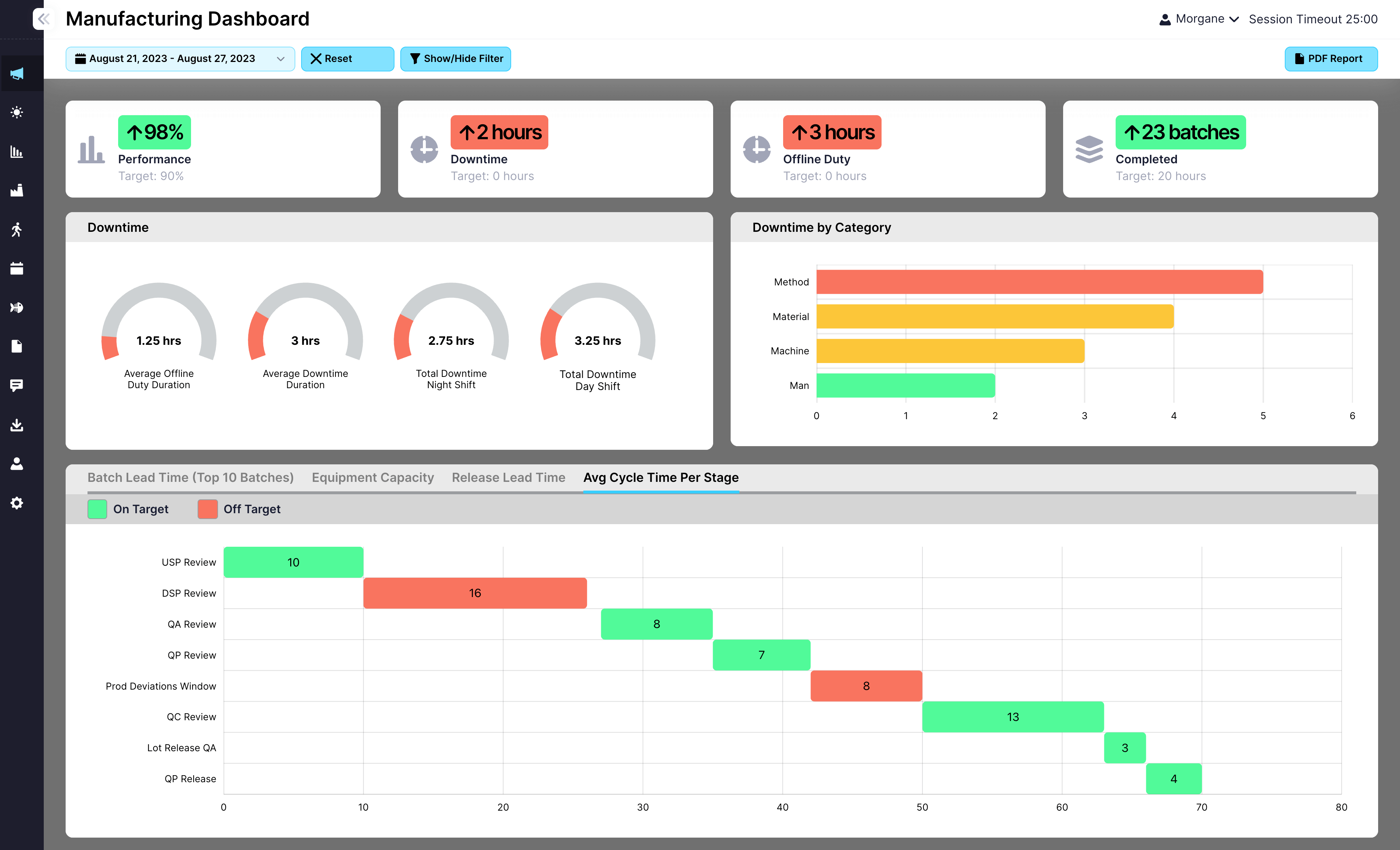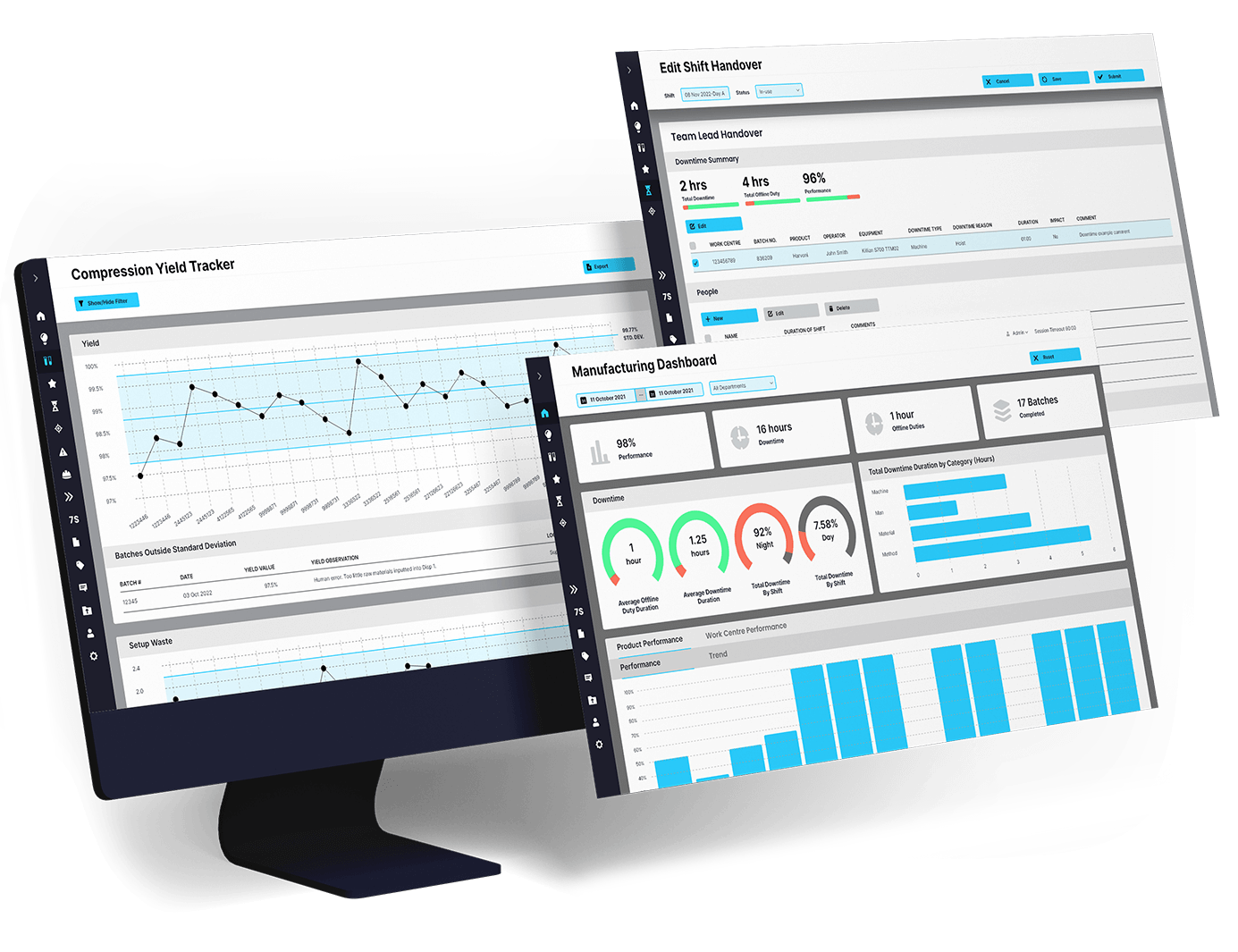
EviView’s batch approval workflow helps teams see every approval step as it happens. From quality checks to paperwork, you can track progress, solve problems early, and keep batches from getting stuck. No paper trails or guesswork, just clear answers and faster release times.
Request a DemoBatch approval workflow fits into your daily work so you never lose track of where a batch stands. As each step is finished, the system updates right away so teams know exactly what still needs to happen before a batch can be released.
Track approvals for quality, safety, and paperwork
See live status of each batch in one place
Spot delays and fix them before they grow
Connect approvals to records, audits, or investigations

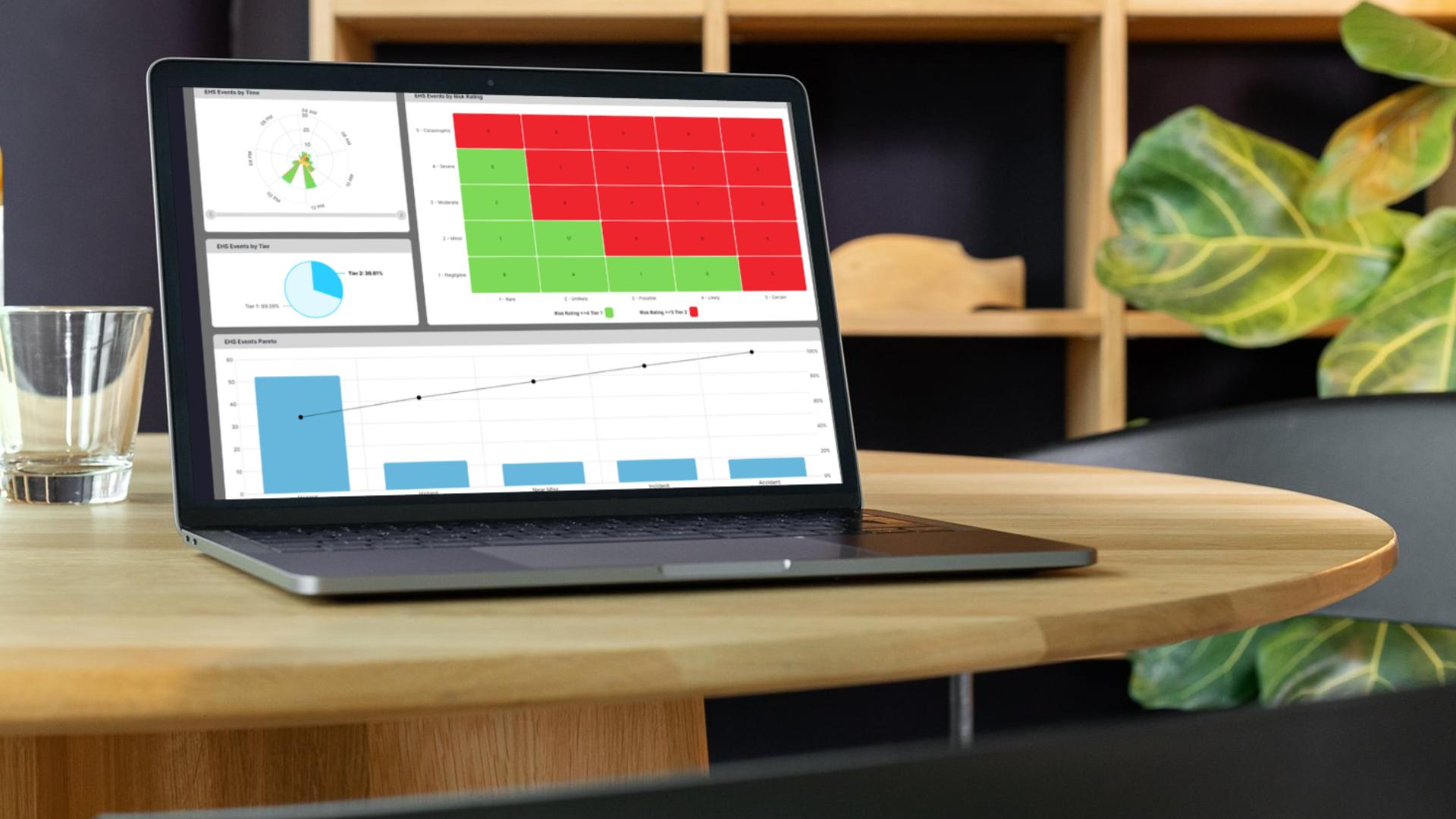
Know exactly where each batch stands and what’s left to do.
Handle approvals without waiting for paperwork to catch up.
Have proof of every step for audits or customer reviews.
Tackle problems before they stop your lines.
Batch Approval Workflow in EviView helps make sure batches don’t sit waiting longer than they should. Teams always know which steps are left, who is responsible, and how close they are to releasing finished goods. Supervisors can look across areas and shifts and jump in if something slows down. This means fewer hold-ups, quicker release times, and better preparation for audits or customer checks. EviView makes batch approval clear and collaborative, so work keeps moving and everyone knows what to expect.
EviView’s batch approval workflow works alongside the tools your teams already use, like MES, ERP, QMS, and production tracking software. Approval updates feed into your existing systems so there’s no double work and no missing details. Teams get reliable data, easier audits, and faster release times without needing to change how they work.
Request a Demo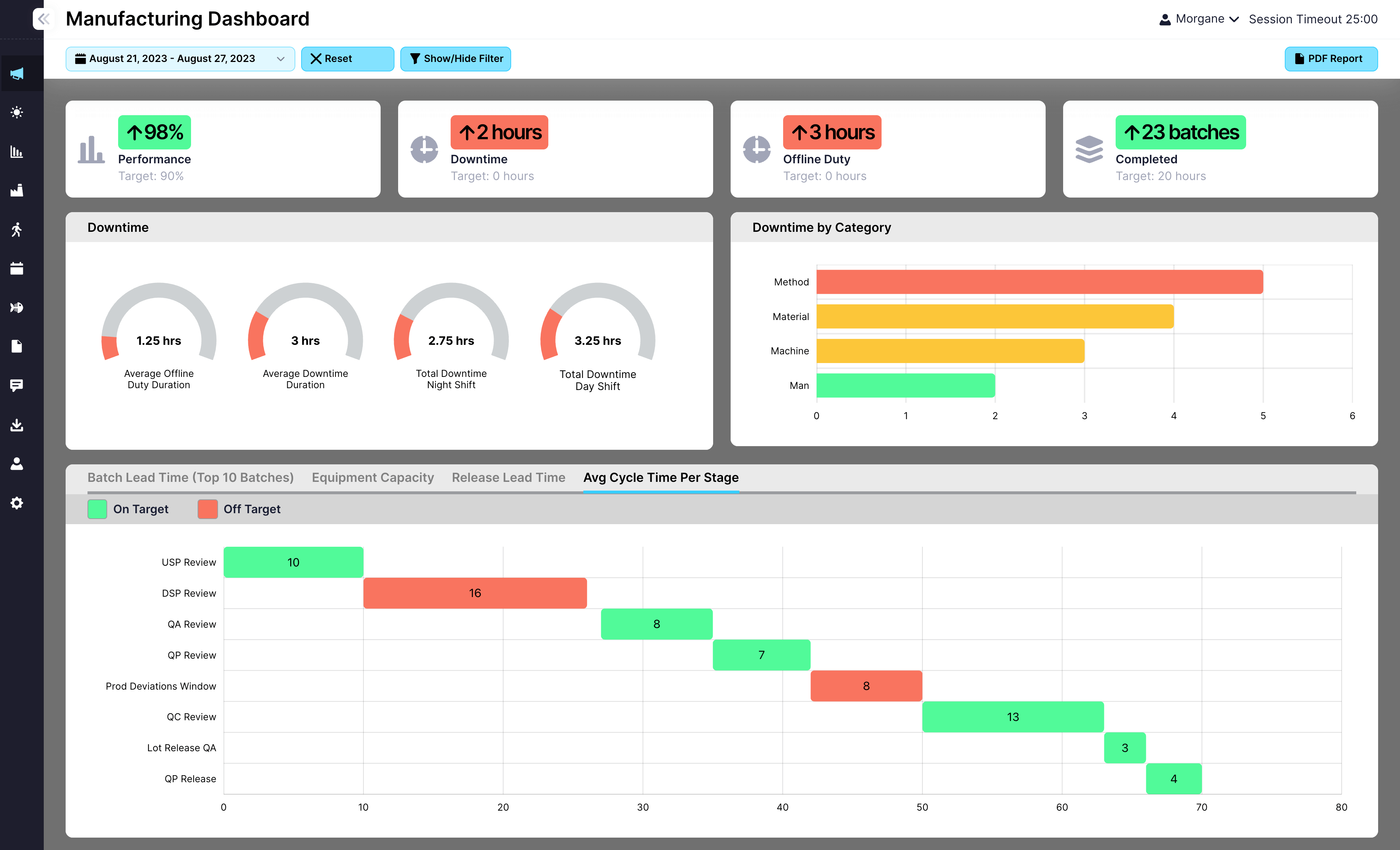
Teams in many industries rely on EviView to keep batch approvals moving from start to finish. Mobile updates, live dashboards, and automatic alerts help everyone stay on the same page and avoid delays.
Here’s how real teams keep products moving without guesswork.

EviView’s Digital Daily Management System has been transformative for Merck. By streamlining shift handovers, centralizing data, and integrating with SAP and Palantir Data Lake, we’ve reduced downtime by 30% and saved €66,000 annually. The system has boosted visibility, improved communication, and increased safety incident reporting by 300%, fostering a culture of continuous improvement and positioning us for long-term success.

Pharmaceutical Company, US
Head of Digitalisation and Strategy

I worked with EviView on an electronic handover project. Their team was very knowledgeable, took time to ensure all stakeholders were kept up to date with the progress of the project, and was always available to give advice on system usage during implementation.

Bio-Pharma Company, US
Manufacturing Team Lead

Karol's team delivered a highly successful change management and digital transformation project. His structured approach, detailed analysis, and process-driven methodology have been invaluable on every project. EviView's understanding of business, IT, operations, and budgetary needs is unmatched.

Top 5 Pharma Manufacturer, EU
Senior Manager of Engineering & Maintenance
EviView connects the work on the floor to the final decision to release a batch. Every check and sign-off is recorded and easy to see. If a step slows down, teams can act fast. If issues repeat, there’s a record to help improve the process. It’s batch approval that fits real production work.