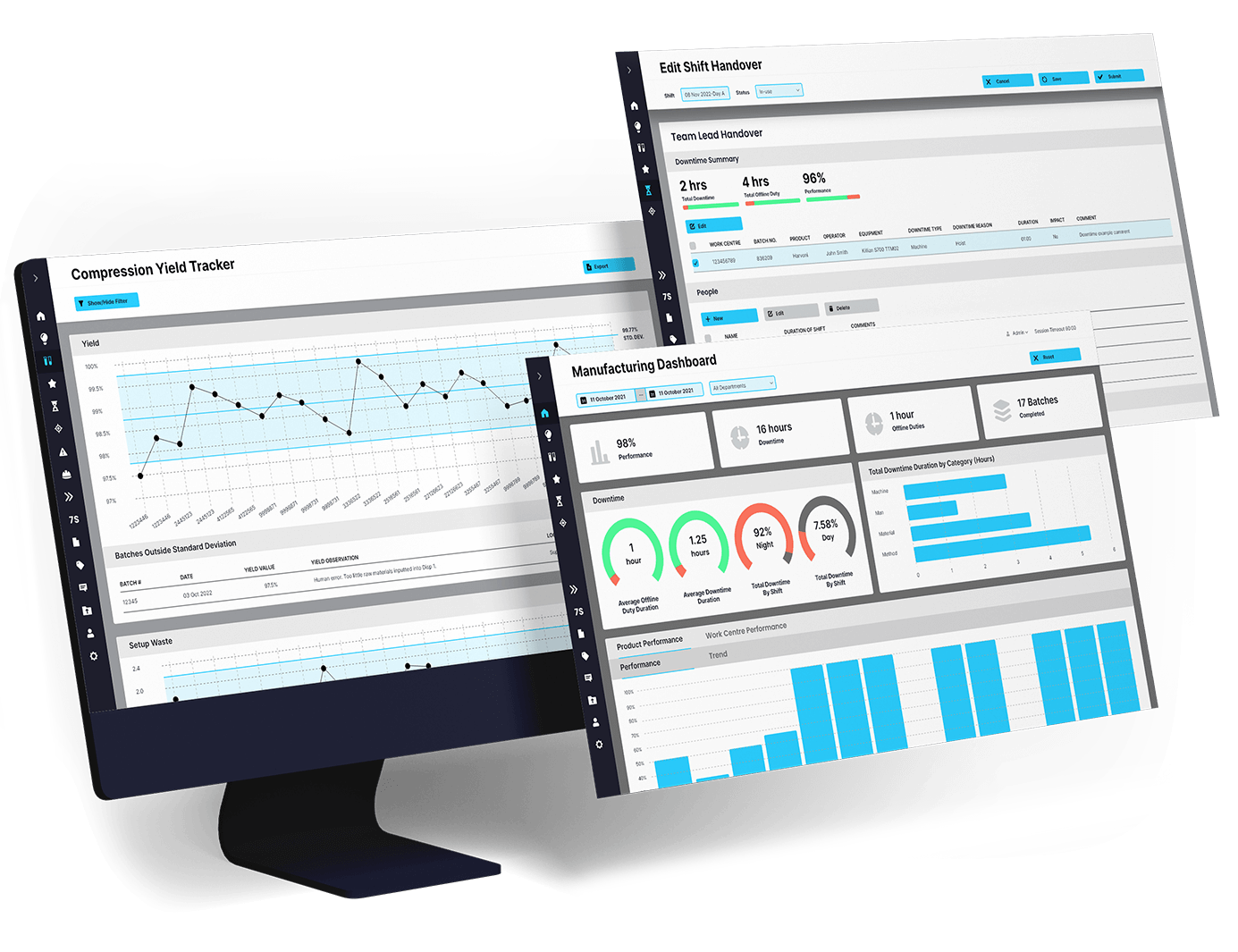
EviView makes post-batch tasks organized and easy to track. Operators, quality teams, and supervisors complete digital checklists directly on mobile devices or workstations, capturing photos, comments, and electronic approvals as tasks are finished.
If tasks are overdue or incomplete, automatic notifications ensure teams can quickly resolve issues and avoid bottlenecks.
Monitor every post-batch task in a digital checklist linked to specific batches
Record digital signatures, timestamps, and attachments for quality and compliance records
Trigger alerts when post-batch activities fall behind schedule
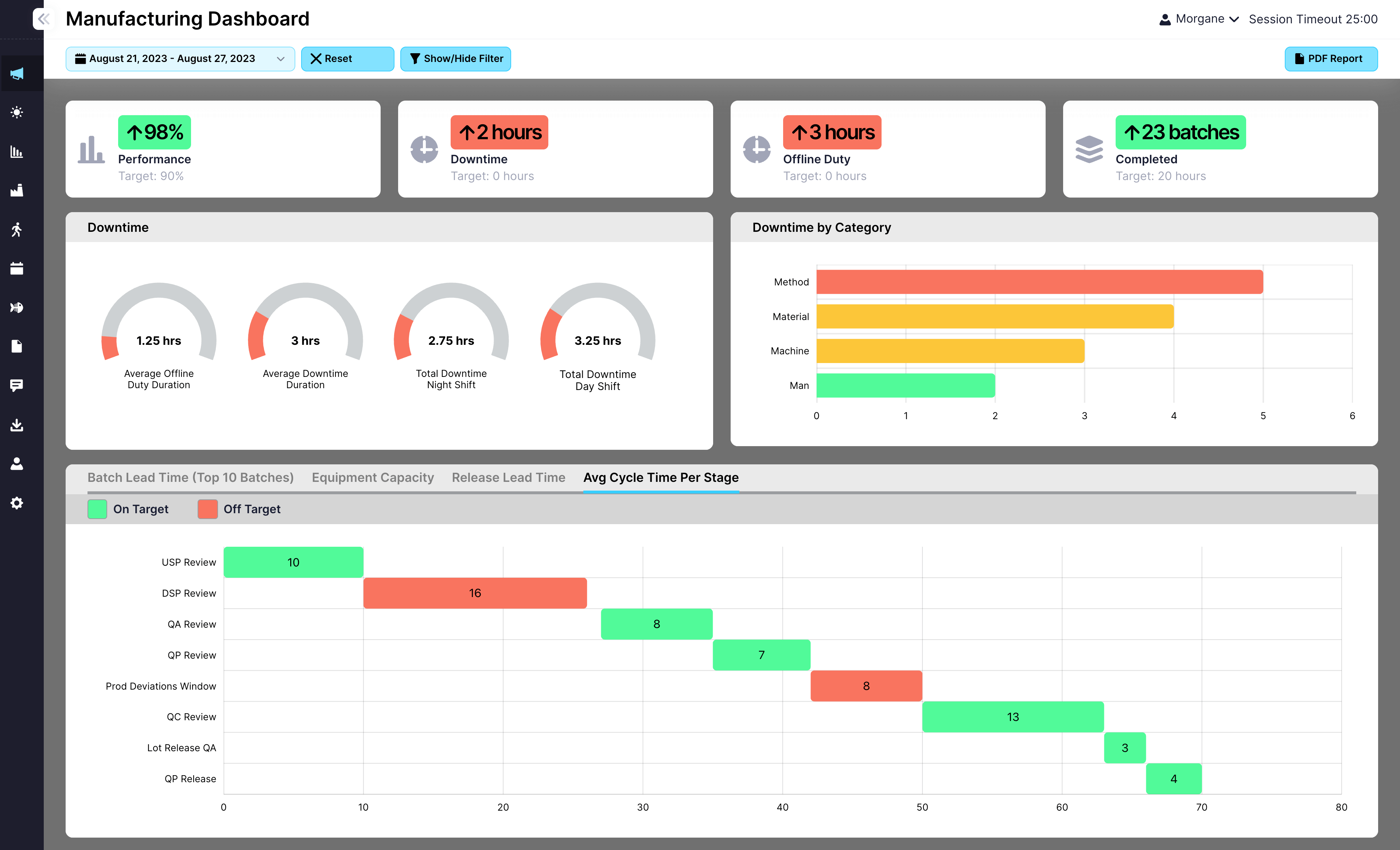
Feed post-batch completion data into dashboards and production KPIs

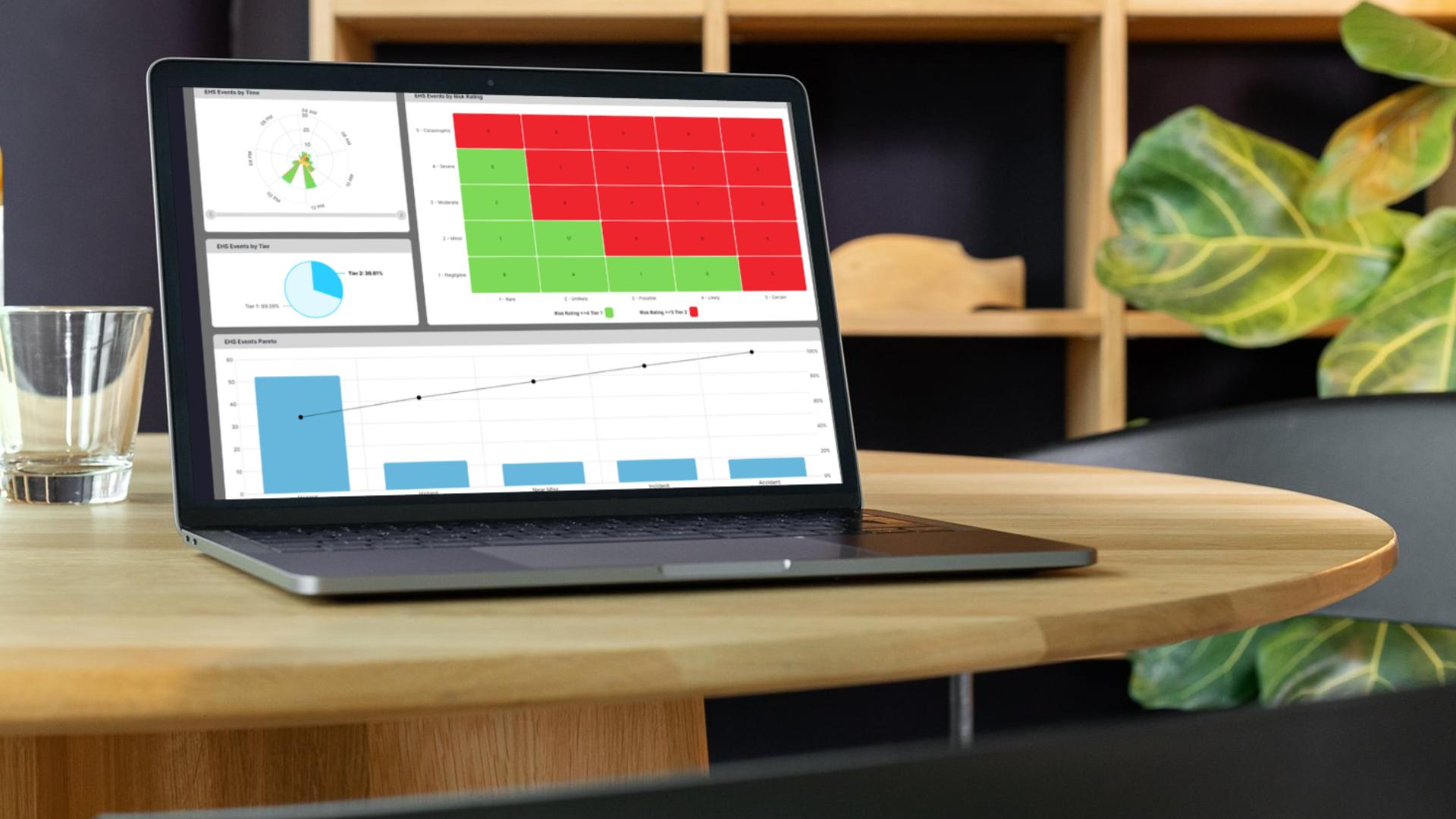
Digital post-batch checks help teams complete cleanups, signoffs, and readiness tasks quickly so lines are ready for the next run.
Digital records ensure all post-batch activities are documented and traceable for audits and regulatory reviews.
Supervisors and managers see real-time progress on post-batch tasks, preventing surprises and delays.
Insights from post-batch data help teams allocate resources effectively and avoid bottlenecks between batches.
EviView’s Post-batch Tracker does more than digitize paperwork. It helps teams close out batches faster, improves compliance, and keeps production flowing. With clear visibility into post-batch activities, manufacturers prevent downtime, avoid quality gaps, and maintain readiness for the next batch.
EviView’s Post-batch Tracker connects seamlessly with MES, ERP, QMS, and EBR systems. Post-batch data flows directly into batch records, compliance reports, and dashboards, eliminating duplicate entry and ensuring fast adoption. Gain audit-ready records and real-time insights into operational readiness across your facility.
Request a Demo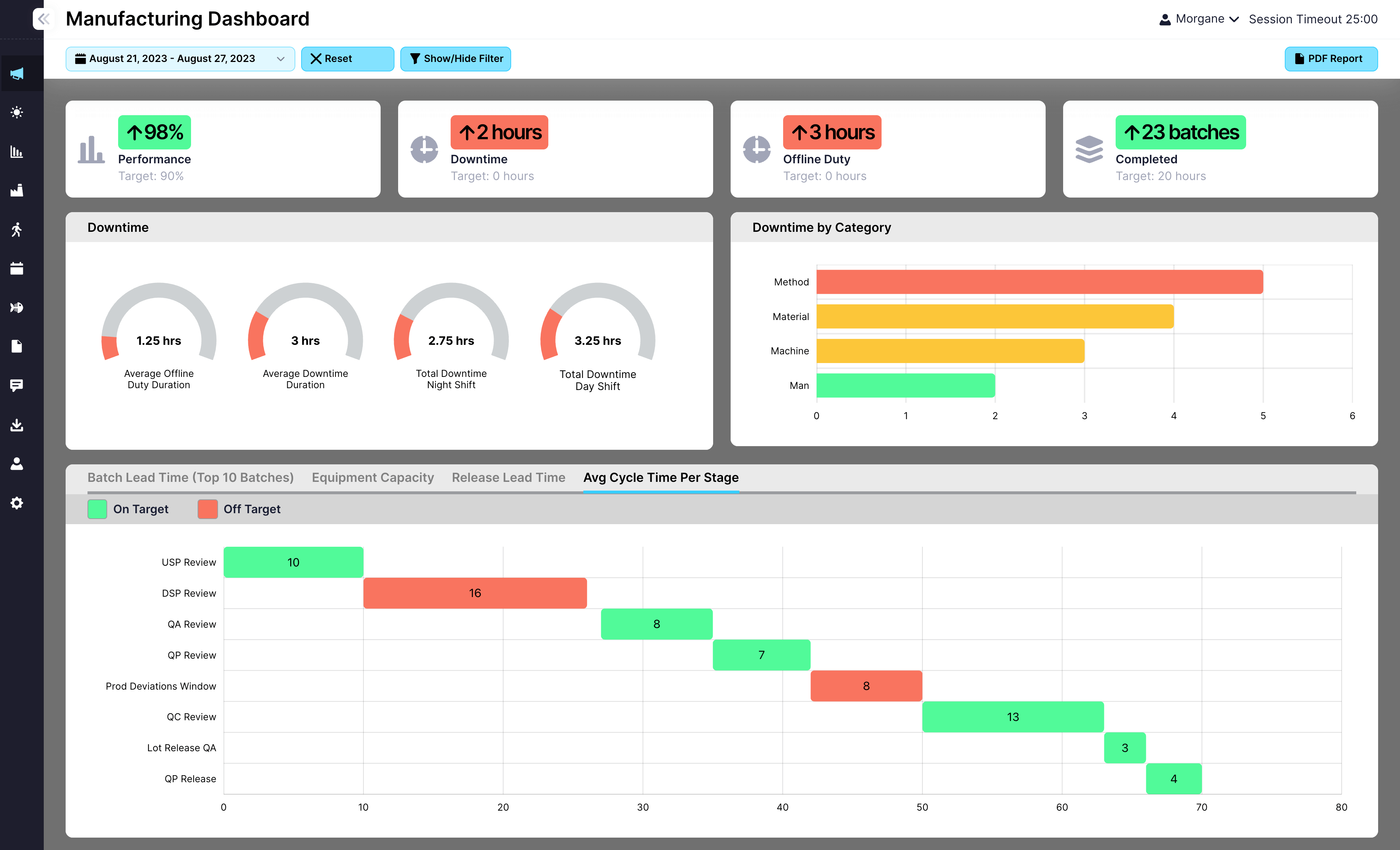
Pharma and manufacturing teams rely on EviView’s Post-batch Tracker to ensure cleanups, inspections, and paperwork happen quickly and completely after every batch. See how our customers use EviView to keep operations moving smoothly.

EviView’s Digital Daily Management System has been transformative for Merck. By streamlining shift handovers, centralizing data, and integrating with SAP and Palantir Data Lake, we’ve reduced downtime by 30% and saved €66,000 annually. The system has boosted visibility, improved communication, and increased safety incident reporting by 300%, fostering a culture of continuous improvement and positioning us for long-term success.

Pharmaceutical Company, US
Head of Digitalisation and Strategy

I worked with EviView on an electronic handover project. Their team was very knowledgeable, took time to ensure all stakeholders were kept up to date with the progress of the project, and was always available to give advice on system usage during implementation.

Bio-Pharma Company, US
Manufacturing Team Lead

Karol's team delivered a highly successful change management and digital transformation project. His structured approach, detailed analysis, and process-driven methodology have been invaluable on every project. EviView's understanding of business, IT, operations, and budgetary needs is unmatched.

Top 5 Pharma Manufacturer, EU
Senior Manager of Engineering & Maintenance
Every post-batch task feeds into EviView’s dashboards, highlighting repeated delays, incomplete steps, and improvement opportunities. Leaders gain real-time insights into where processes can be streamlined to keep lines ready for the next batch. Post-batch tracking becomes a powerful tool for driving operational efficiency and compliance.