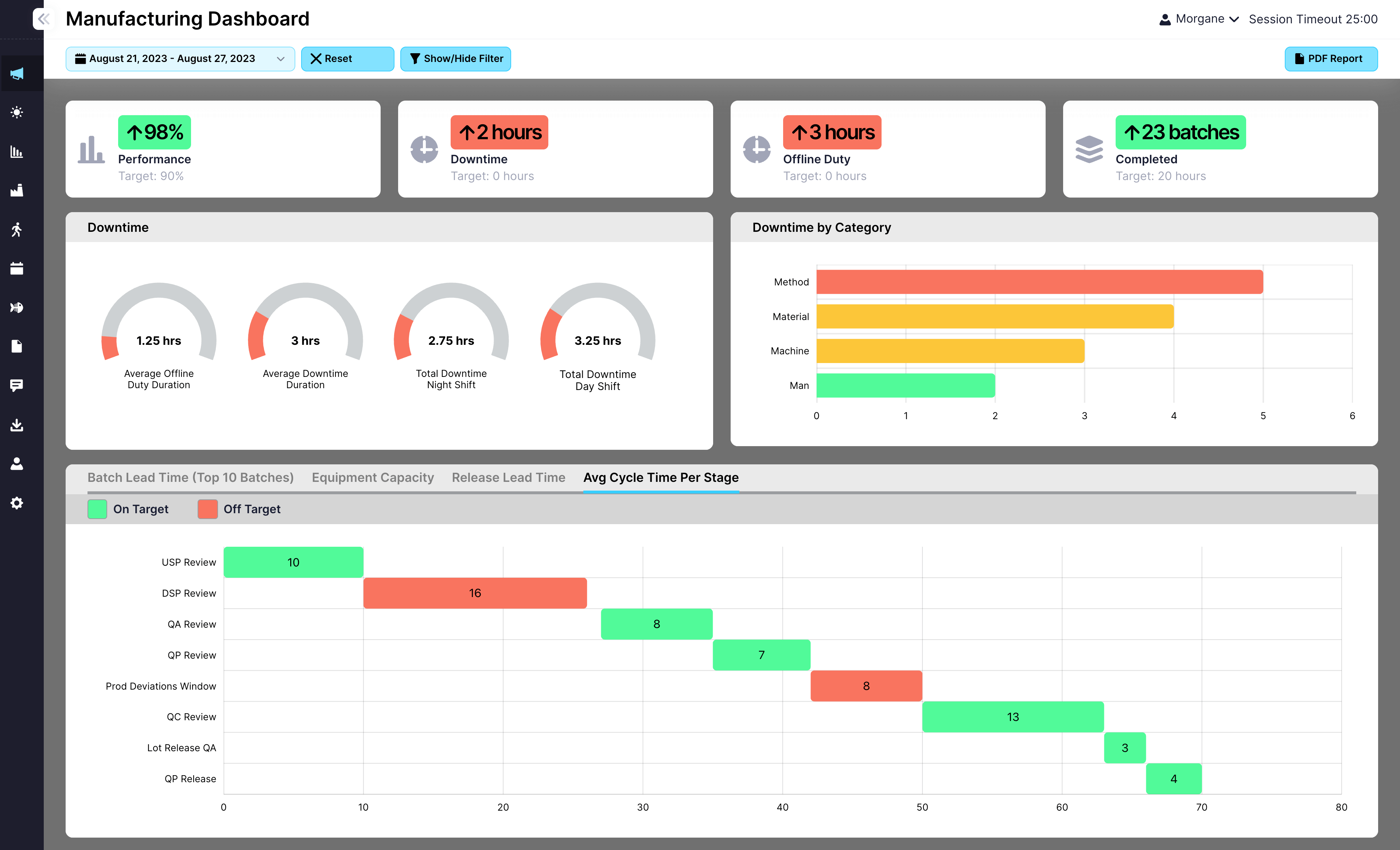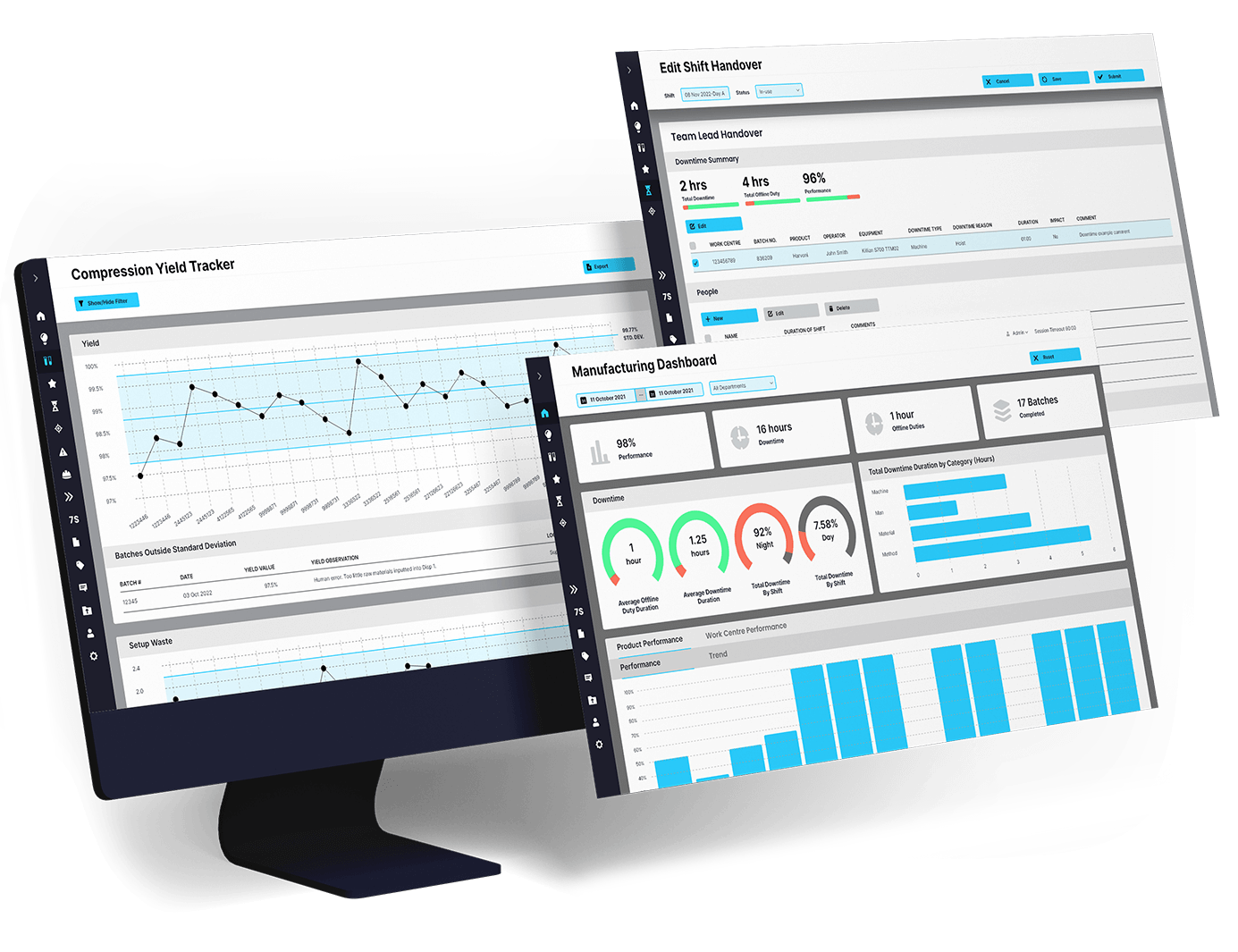
When quality issues span shifts, missing information leads to missed compliance, slow response, or recurring deviations. EviView makes quality shift handover simple and traceable, giving QA teams a clear, connected process to log updates, flag open items, and keep investigations moving without interruption.
Request a DemoQuality teams don’t need more paperwork, they need a structured, digital workflow that aligns with production and QA schedules. EviView gives them a purpose-built system to log quality observations, open deviations, pending CAPAs, or in-process reviews, all in one place, and accessible across shifts.
Document quality updates with timestamp, product, and batch details
Log ongoing deviations and link them to related shift data
Flag unresolved issues and hand off pending QA decisions
Maintain digital traceability across team members, areas, and departments

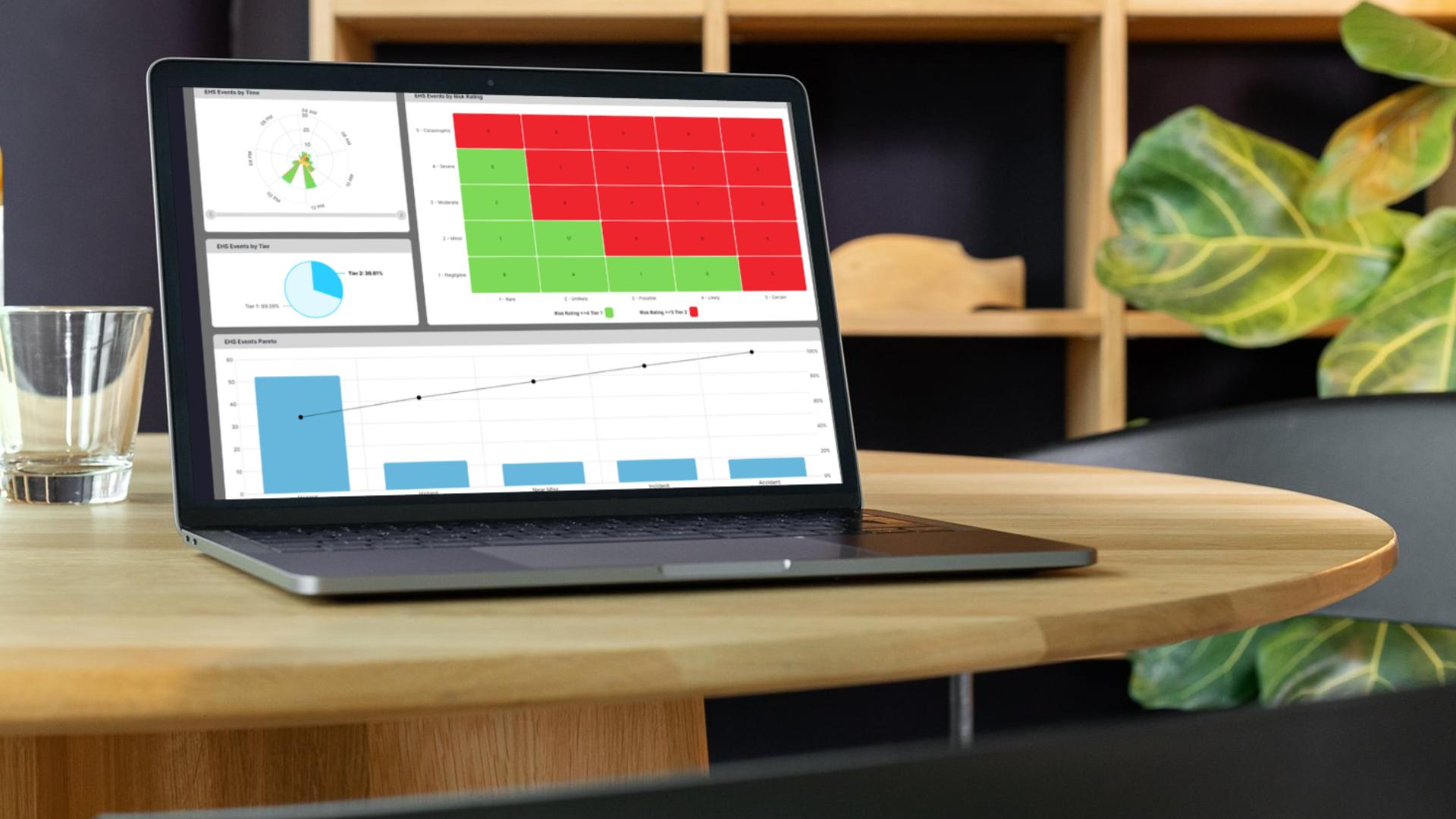
Give quality teams consistent records and full traceability from shift to shift with no blind spots.
Surface quality issues early and share progress updates instantly, no delays in investigation or closure.
Let teams share relevant context, data, and next steps, without bouncing between systems or channels.
Log shift activity with full attribution, time stamps, and links to CAPAs, investigations, and follow-ups.
Inconsistent quality handovers create gaps that put compliance and performance at risk. EviView replaces handwritten notes and offline trackers with a digital workflow built for accuracy, clarity, and compliance. When updates are structured, traceable, and easy to share, teams avoid miscommunication and resolve quality issues faster. Here’s what teams gain when quality shift handovers are digitized, connected, and aligned with operations.
Most digital tools weren’t designed with shift-based QA in mind, but EviView is. It brings quality and production closer, connecting shift observations to investigations and action tracking, so work doesn’t stall waiting for information or approvals.No toggling between paper, email, and spreadsheets, just one connected flow where quality actions stay visible and accountable until resolved.
Request a Demo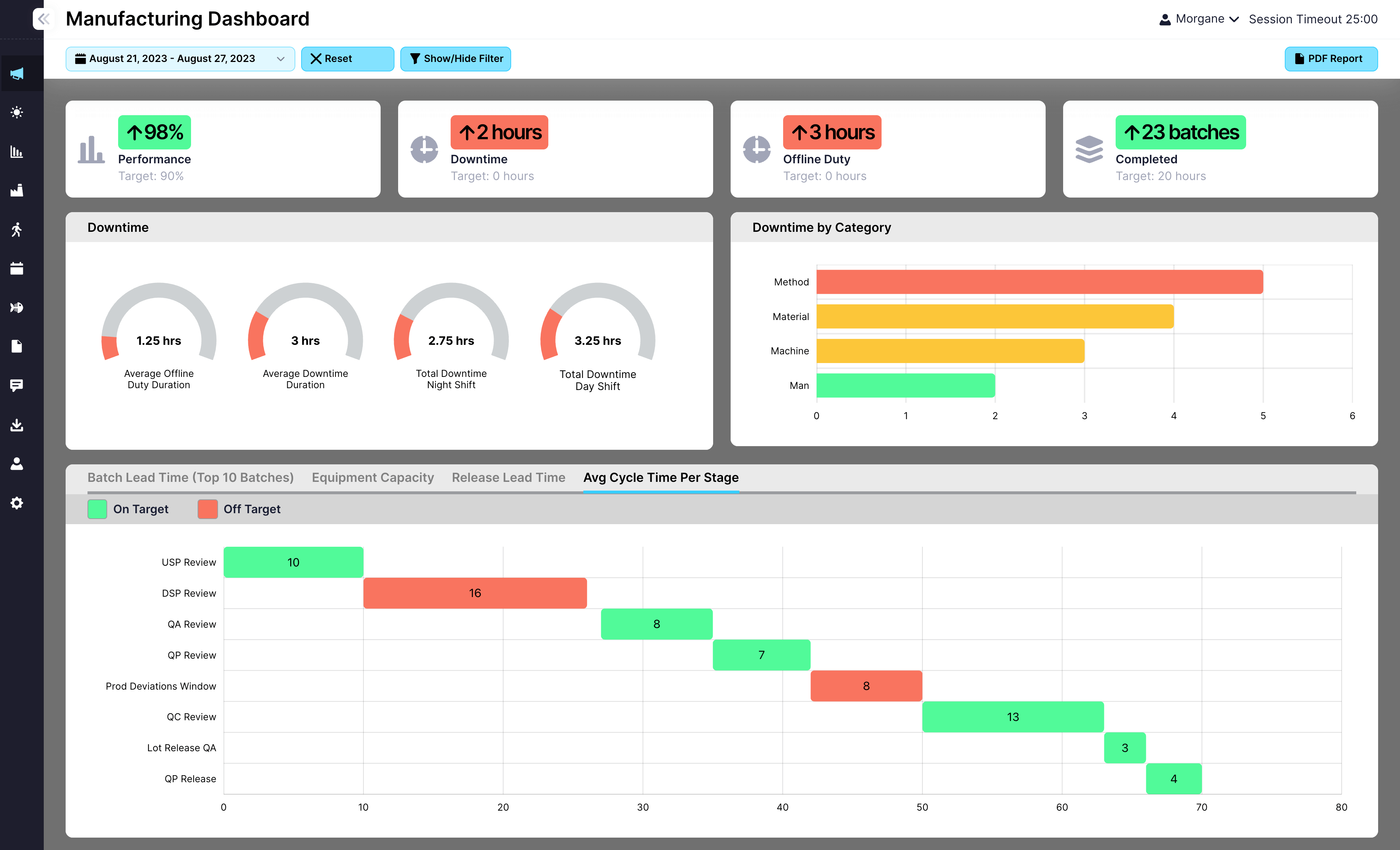
Quality shift handovers affect everyone from floor-level inspectors to compliance leaders. Here’s how real manufacturing teams use EviView to improve their process, and what they say about the difference it makes.

EviView’s Digital Daily Management System has been transformative for Merck. By streamlining shift handovers, centralizing data, and integrating with SAP and Palantir Data Lake, we’ve reduced downtime by 30% and saved €66,000 annually. The system has boosted visibility, improved communication, and increased safety incident reporting by 300%, fostering a culture of continuous improvement and positioning us for long-term success.

Pharmaceutical Company, US
Head of Digitalisation and Strategy

I worked with EviView on an electronic handover project. Their team was very knowledgeable, took time to ensure all stakeholders were kept up to date with the progress of the project, and was always available to give advice on system usage during implementation.

Bio-Pharma Company, US
Manufacturing Team Lead

Karol's team delivered a highly successful change management and digital transformation project. His structured approach, detailed analysis, and process-driven methodology have been invaluable on every project. EviView's understanding of business, IT, operations, and budgetary needs is unmatched.

Top 5 Pharma Manufacturer, EU
Senior Manager of Engineering & Maintenance
EviView makes it easy to turn shift-level quality notes into tasks with real follow-up. When an issue is flagged—whether it’s an audit observation, a spec deviation, or a pending batch release, it doesn’t just sit in a log. It gets assigned, time-stamped, and connected to the right workflow.